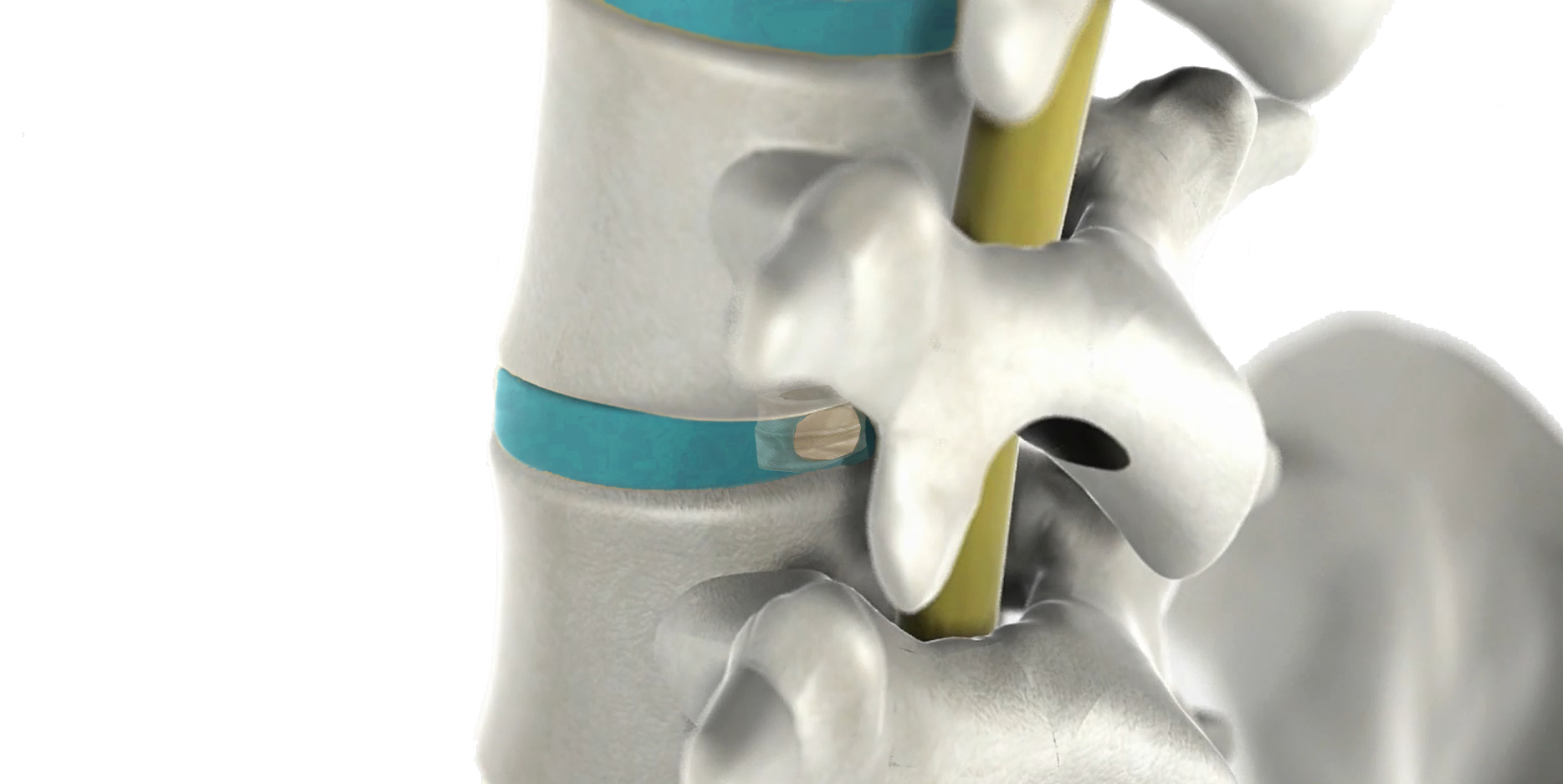
The resources obtained will help solve the problem of recurrent lumbar disc herniation.
NEOS Surgery, a technology-based company dedicated to developing, manufacturing and marketing medical devices for the neurosurgery sector, has received a non-refundable grant of 1.9 million euros from the European Innovation Council EIC Accelerator ), a European programme for funding innovative SMEs.
The company was founded in 2003 thanks to a collaboration between Eurecat and TECNALIA, and supported by the Deep Tech Venture Builder TECNALIA Ventures. It will use the resources obtained to clinically validate the DISC Care™ device, which will make it possible to solve the problem of recurrent lumbar disc herniation. This funding also ensures that NEOS Surgery can generate additional private investment to bring this technology to European patients.
EIC Accelerator selects the most innovative SMEs with high growth potential to help them develop and launch new disruptive products, services and business models onto the market. More than 4,200 projects from 18 countries were submitted under the last call for grants, of which only 38 received funding.
Quality of life of patients with herniated discs
According to Lluís Chico, CEO and partner of NEOS Surgery, vice-chairman of CataloniaBio & HealthTech and member of the Health Engineering Committee of the Official Association of Industrial Engineers of Catalonia (COEIC), “this European Commission aid underlines the innovative character and the strong technological basis of NEOS Surgery. This grant will undoubtedly provide key funding to accelerate the study on the safety and effectiveness of the DISC Care™ device. We are convinced that this is a medical solution that will help to improve the quality of life of patients with herniated discs”.
The aim of NEOS Surgery is to complete the clinical trial of the DISC Care™ device at five Spanish university hospitals during 2021 and then enter the regulatory phase in Europe and the United States and start marketing the product in 2023. The company has the Cranial LOOP™ and Cranial COVER™: devices on the market: they are used for finishing off cranial surgery without the need for medical instruments. It also has another innovative device for cardiac surgery.
New investor in Neos Surgery
Avançsa, an organisation run by the Catalan Ministry of Enterprise and Knowledge, which promotes industrial innovation, recently joined Neos as an investor.